Earlier posts provided a brief introduction to Our Beautiful Bones as well as tips on How to Promote Healthy Bones. In short, a balanced system of remodeling maintains bone health. When osteoblast (bone building) and osteoclast (bone deconstruction) activity get out of synch, bones become more fragile and elevate the risk for fracture.
Dr. R. Keith McCormick, author of The Whole Body Approach to Osteoporosis, wrote a fascinating article that ties bone health to immune function.1 This high-level summary seeks to capture the main concepts for laypersons (as I understand them!) We’ll start by looking at the genesis of osteoclasts and osteoblasts.
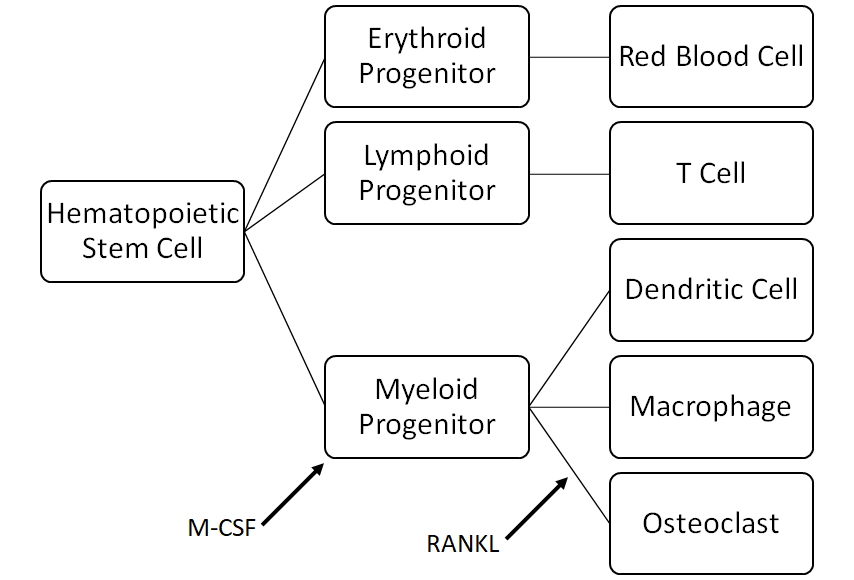
Osteoclasts trace their origin to hematopoietic (blood-oriented) stem cells located in the bone marrow. These stem cells can differentiate into:
- Red blood cells, which carry fresh oxygen all over the body
- T Cells, which help the body mount an adaptive immune response
- Dendritic cells, which are responsible for initiating the adaptive immune response
- Macrophages, which engulf and digest cancer cells, pathogens, cellular debris, and other foreign/unhealthy substances
- Osteoclasts, which secret acid phosphatase to dissolve bone crystal (hydroxyapatite)
The presence of macrophage colony-stimulating factor (M-CSF) causes the hematopoietic stem cell to differentiate into myeloid progenitors. M-CSF may be released by osteoblasts (bone builders) in response to stimulation by the parathyroid; it may also be released when the body senses a need to combat inflammation. Osteoclasts come into being when RANKL attaches to the receptor activator of nuclear factor KB (RANK) on the myeloid progenitor.
Note that RANKL does not confine its activities to bone remodeling; it also plays a role in immune function. It can be expressed by Helper T Cells to activate B cells to secrete antibodies and macrophages, or activate cytotoxic T cells. It may also alert the immune system to lymph-born antigens.
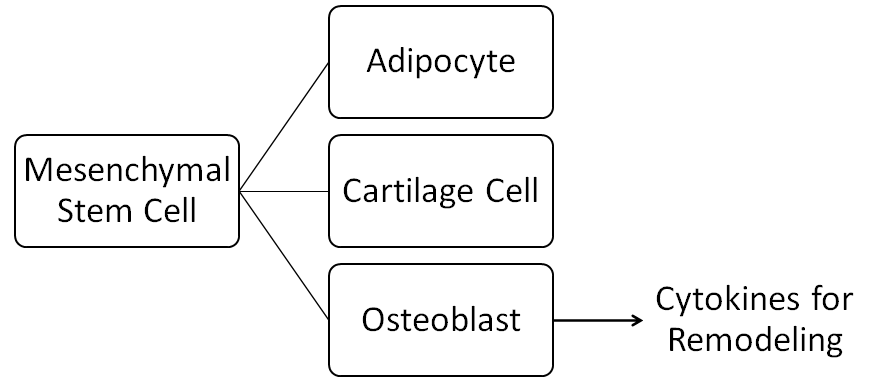
Osteoblasts trace their origin to mesenchymal stem cells. These stem cells can differentiate into:
- Adipocytes, which specialize in storing energy as fat
- Cartilage cells, which form connective tissue found in the larynx and respiratory tract, the external ear, and in the articulating surfaces of joints
- Osteoblasts, which leverage cytokines (cell signaling proteins) to instigate bone remodeling and subsequently deposit collagen and alkaline phosphatase into bone excavation sites for mineralization
In healthy individuals, osteoblasts actuate just the right amount of messenger proteins (e.g., RANK-L) to dissolve bone in a manner consistent with the osteoblasts’ capacity for bone building. Osteoblasts also produce osteoprotegerin (OPG), a soluble decoy receptor that absorbs excess RANK-L and keeps it from activating too many osteoclasts.
The RANK/RANKL/OPG system of bone homeostasis receives substantive bone remodeling support from estrogen. Estrogen improves Vitamin D absorption in the gut (for improved availability of bone-building minerals) and stimulates the release of calcitonin (to make osteoclasts less active). Moreover, estrogen-receptor activation of osteoblasts stimulates release of the growth factors TGF-β and IGF-1, and OPG. This action limits M-CSF and RANKL (which reduces osteoclast formation) and increases osteoclast cellular death.
Individuals with reduced estrogen levels (notably post-menopausal women) and/or persistent activation of the immune system may lack the natural ability to limit RANKL engagement in osteoclast production. As noted previously, both the immune and bone remodeling systems use the same intercellular communications tools. Unfortunately, osteoclast precursors do not consider the source of the signals when acting upon them. When immune system activation boosts production of RANKL and cytokines, the healthy balance of osteoclast-osteoblast function tips in favor of bone breakdown and compromises bone strength, density, and microarchitecture. Therefore, reducing antigenic load, inflammation, and oxidative stress may prove as critical for sustaining bone health as estrogen.
Many of us are unaware of the ways in which we activate our immune systems. We may feel that our bones are not at risk because we don’t have inflamed tissues, viral infections, or other obvious signs of physical distress. And yet our lifestyles may induce immune responses that fly “under the radar.” For example:
- Oxidative stress is stimulated by processed food, preservatives, food coloring, air pollution, toxins, and smoke inhalation.
- A weak intestinal endothelial lining (a.k.a. “leaky gut”) may permit bacteria and dietary antigens to escape into the blood stream. Common factors that increase intestinal permeability include: alcohol, food allergies, gluten, NSAIDs (e.g., Advil, Motrin, ibuprofen), psychological stress, surgery/trauma, and unsaturated fats.
- A high sugar diet may encourage bacterial and/or fungal overgrowth in the gastrointestinal track and oral cavity, such as the characteristic white-coated tongue associated with Candida albicans.
- Chronic inflammation caused by long-term infection (e.g., periodontal disease), food allergies, autoimmune disorders, and the like evoke immune response.
- Chronic stress amps up inflammation and weakens immune function.
Dietary and specific nutrient interventions can reduce inflammation and limit the potential impact of excess osteoclastic activity. Ask your doctor for recommendations, or read Dr. McCormick’s article.
1 R. Keith McCormick, DC, CCSP, Osteoporosis: Integrating Biomarkers and Other Diagnostic Correlates into the Management of Bone Fragility, Alternative Medicine Review, Volume 12, Number 2, 2007